There are FOUR Cochlear Implant manufacturers in the UK. These are Advanced Bionics, Cochlear, MED-EL and Oticon Medical. Each company offers a selection of implants and processors.
There is one company investigating the potential of cell therapy to treat hearing loss: Rinri Therapeutics

About ADVANCED BIONICS
At Advanced Bionics, we believe everyone can live without the limitations of hearing loss.
For 30 years, we’ve been developing leading hearing innovations that help people hear again, or for the first time. Our partnership with Phonak allows us to provide even more leading-edge hearing solutions through our integration with the world’s most advanced and trusted power hearing aids.
Advanced Bionics provides a comprehensive range of products designed to meet your needs and your lifestyle. Our cochlear implant system offers more ways to hear than any other CI. And every aspect of our CI has been optimised for clarity, comfort, ease of use, and reliability—allowing you to hear your best.
If you are an Advanced Bionics cochlear implant wearer or are considering cochlear implants for yourself or a loved one, you will find one-to-one support from AB.
For more information:
info.uk@advancedbionics.com
www.advancedbionics.com or book a one-to-one appointment www.AdvancedBionics.com/CWS121
Advanced Bionics Technology
HiRes™ Ultra 3D Cochlear Implant
Designed for optimal hearing experience and hassle free MRI
Sophisticated yet incredibly strong, the award-winning HiRes™ Ultra 3D cochlear implant delivers the proven benefits of clearer speech1 and a broader range of sound2. It is designed to exceed industry standards for impact resistance3, allowing you to enjoy life’s adventures without hesitation. With the HiRes Ultra 3D implant you can enjoy hassle free and pain free MRI examinations, with uninterrupted hearing.
*The HiRes Ultra 3D Cochlear Implant has been awarded the Medtech Breakthrough Award 2019 for Best Medical Device Solution.
HiFocus™ Mid-Scala Electrode
Designed for optimal cochlear placement
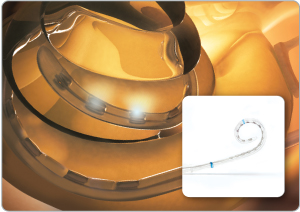
The industry’s latest innovation in electrode design. Developed through extensive research and using state-of-the-art manufacturing processes, the HiFocus Mid-Scala electrode has been designed for optimal placement in the cochlea to protect its delicate structures and preserve any residual hearing.
Naída™ CI M Sound Processor 
Designed with you in mind
Naída CI M is a part of an innovative, advanced cochlear implant system that delivers a brilliant hearing experience. Naída CI M offers a wide variety of features designed to improve hearing across all listening situations using AutoSense OS™ 3.0 operating system and direct streaming from a multitude of devices. Rest assured—every aspect of the system is designed to be seamlessly operated, providing you the ultimate ease of use.
For unique environments such as the swimming pool, noisy restaurants, or meetings, there are a variety of accessories designed to help you hear your best and be at the centre of the conversation.
For individuals with some hearing in one ear, wearing the right hearing aid helps them to hear even better than with a CI alone. Wearing a Naída Link M together with Naída CI M gives you a consistent sound impression between ears, and allows you to adjust both devices with a single control.
More information
Sky CI™ M Sound Processor 
Designed with children in mind
Sky CI M is part of an innovative, advanced cochlear implant system that delivers a brilliant hearing experience for your child. Sky CI M offers a wide variety of accessories that you and your child can use for specific needs—from listening in the classroom to laughing when swimming and beyond. With so many features available, rest assured that every aspect of the system is designed to operate seamlessly, providing the ultimate in ease of use.
Our waterproof battery delivers the same performance even during the most rugged childhood activities—all while providing secure, worry-free wearing. Advanced Bionics was the first to offer waterproof microphones and headpieces. Our Slim HP AquaMic™ headpiece maintains a great hearing experience for your child while they play in the pool with their friends.
Discover more
Advanced Bionics Support
AB Community Mentors
Here to support you on your hearing journey
There really is nothing like talking to someone who knows what life with a cochlear implant is like. Through AB Community Mentors, you can directly connect with an AB cochlear implant wearer, parent or family member who can assist you in navigating the process of choosing and living with cochlear implants. If you’d like to connect with your local AB Community Mentor email hear-uk@AdvancedBionics.com You can also connect with an AB community mentor on the HearingJourney™ online forum.
Ongoing Education and Rehabilitation
Maximise your hearing potential
Advanced Bionics recognises that this is a life-long journey—one on which we’ll accompany you every step of the way. HearingSuccess resources can help you identify your hearing needs, set goals, and get support. Stay up to date on the latest developments in technology by utilising powerful tools and resources that can assist with your HearingSuccess. Learn more
Communicate with Success Events
Join us at an upcoming event or book a virtual one-to-one appointment
Are you considering cochlear implants for yourself or loved one? Or do you already have an Advanced Bionics cochlear implant? Join us at a Communicate with Success event and learn more about AB technology and support. Visit www.AdvancedBionics.com/uk-events for the latest events. Your AB team is also available for one-to-one support, you can book a virtual appointment at a time that is convenient for you, online at: www.AdvancedBionics.com/CWS121
Contact Us
+44 (0) 1223 847 888
info.uk@advancedbionics.com
Useful links
Website: www.AdvancedBionics.com
Events Calendar: www.advancedbionics.com/uk-events
HearingJourney™: www.HearingJourney.com
Facebook: www.facebook.com/AdvancedBionics
Twitter: www.twitter.com/AdvancedBionics
YouTube: www.YouTube.com/AdvancedBionics
LinkedIn: www.linkedin.com/company/advanced-bionics
Instagram: www.instagram.com/AdvancedBionics
References:
- Koch DB, Osberger MJ, Segel P, Kessler DK. (2004) HiResolution and conventional sound processing in the HiResolution Bionic Ear: using appropriate outcome measures to assess speech-recognition ability. Audiology and Neurotology, 9:214-223.
- Firszt JB, Koch DB, Downing M, Litvak L. (2007) Current steering creates additional pitch percepts in adult cochlear implant recipients. Otology and Neurotology, 28(5):629-636.
- EN 45502-2-3:2010. Active Implantable Medical Devices. Particular Requirements for Cochlear and Auditory Brainstem Implant Systems.

Cochlear. Hear Now. And always
Cochlear is the global leader in implantable hearing solutions. We have provided more than 750,000 hearing implants worldwide, helping people in more than 180 countries to hear and connect with life’s opportunities. Perhaps we could help you or a loved one too.
If you are considering a cochlear implant, you can be confident in choosing Cochlear. Our next-generation hearing solutions help people of all ages to achieve their best possible hearing. In addition, we offer tailored support at every stage of the hearing journey. No wonder more people choose Cochlear than any other hearing implant company.
Find out more at:
www.cochlear.com
Cochlear Implants
The Cochlear™ Nucleus® Nexa™ System
The future of hearing. Delivered today

The Nucleus® Nexa™ System is the world’s first and only smart cochlear implant. Designed to support future technologies as they emerge, our smart implant ensures you or your child are always ready to access new features and innovations through a simple firmware update.
The Nucleus Nexa System is also the only cochlear implant with internal memory. This means your unique hearing information is always with you and can be transferred quickly and easily to a new Nucleus Nexa sound processor in the future.
What's more, when you choose Cochlear you are choosing the most reliable cochlear implants available.1
Discover more
Sound Processors
Designed to help you hear your best and engage with the moments that matter, Cochlear sound processors deliver proven hearing performance2-8 through our latest technologies. We offer both behind-the-ear and off-the-ear sound processors, suitable for all ages.
Smaller. Smarter. Better connected.‡
Explore more possibilities with the world’s smallest and lightest behind-the-ear sound processor.9 The Nucleus 8 Nexa Sound Processor brings together next-generation Bluetooth® connectivity and smarter hearing performance, so you can experience your best hearing.2-7, 10-12
Designed to help you communicate with people more easily, the Nucleus 8 Nexa Sound Processor senses changes in your environment and automatically adjusts your listening settings help you to hear more clearly wherever you go.
Cochlear™ Nucleus® Kanso® 3 Nexa™ Sound Processor
Connect. Focus. Explore.
Smart has never been so simple
 Connect to what you love, focus on what matters and explore the freedom of the world’s smallest, lightest and most advanced rechargeable off-the-ear sound processor.9,13
Connect to what you love, focus on what matters and explore the freedom of the world’s smallest, lightest and most advanced rechargeable off-the-ear sound processor.9,13
The Nucleus® Kanso® 3 Nexa™ Sound Processor features intuitive hearing technology that adjusts your listening settings automatically, to help you hear your best, even in challenging environments.4-6, 14 Ready for next-generation Bluetooth LE Audio connectivity, the Kanso 3 Sound Processor also offers you sound in more places and from more devices than ever before.10-12,^
Discover more
Personal support, tailored for you
At Cochlear, we understand that making decisions about hearing health can feel overwhelming – but you don’t need to go through it alone. We are here to answer your questions, and to guide you through your hearing implant journey. We even provide opportunities to connect with Cochlear volunteers, who will share their personal stories. So whether you’re navigating this decision for yourself or as a parent, we are here to help every step of the way.
Connect with us today
Cochlear Family
Cochlear Family is at the heart of our commitment to lifetime support. Designed to help guide recipients through their cochlear implant journey, Cochlear Family offers easy access to a supportive and inspiring global community of people. Cochlear Family provides a range of resources and benefits which together could help you reach your full hearing potential.
Join the family here
Contacting Cochlear
- Cochlear’s UK and Ireland team are here to help.
- If you are considering a cochlear implant for yourself or a loved one, you can find information and support here:
https://www.cochlear.com/uk/en/campaign/hear-more-clearly-again
hearnow@cochlear.com
- If you already have a cochlear implant and need to contact our Customer Service Team:
uksupport@cochlear.com
Tel:01932 263640
References
1 D1932780. Cochlear Nucleus Reliability Report, Volume 20 December 2021.
2 Cochlear Limited. D1864200 SCAN-X Design Description. Apr 2022.
3 Warren C, Nel E, and Boyd P. Controlled comparative clinical trial of hearing benefit outcomes for users of the Cochlear™ Nucleus® 7 Sound Processor with mobile connectivity. Cochlear Implants International (2019 Feb); 20(3)
4 Mauger SJ, et al. Clinical evaluation of the Nucleus 6 cochlear implant system: performance improvements with SmartSound iQ. International Journal Of Audiology. 2014, Aug; 53(8): 564 576. [Sponsored by Cochlear]
5 Mauger SJ, et al. Clinical outcomes with the Kanso™ off-the-ear cochlear implant sound processor. International Journal of Audiology. 2017 Apr 3;56(4):267-76. [Sponsored by Cochlear]
6 Wolfe J, et al. Benefits of Adaptive Signal Processing in a Commercially Available Cochlear Implant Sound Processor. Otol Neurotol. 2015 Aug;36(7):1181-90.
7 Cochlear Limited. D1660797. CP1150 Sound Processor Interim Clinical Investigation Report. 2020
8 Sivonen V, Willberg T, Aarnisalo AA, Dietz A. The efficacy of microphone directionality in improving speech recognition in noise for three commercial cochlear-implant systems. Cochlear Implants Int. 2020 May;21(3):153-159.
9 Cochlear Limited. D1190805 Processor Size Comparison. March 2024
10 Hunn N. Introducing Bluetooth® LE Audio [Internet]. [cited 2022 Jan]. Available from: https://www.bluetooth.com/learn-about-bluetooth/recent-enhancements/le-audio/
11 Cochlear Limited. D1631375 Nucleus 8 Sound Processor Product Definition
12 A Technical Overview of LC3 [Internet]. Bluetooth® Technology Website. [cited 2022 Feb 28]. Available from: https://www.bluetooth.com/blog/a-technical-overview-of-lc3
13 Cochlear Limited. D1930947 – CP1170 and CP1175 Sound Processor Product Specification. 2023, Dec.
14 Jones M, Warren C, Mashal M, Greenham P, Wyss J. Speech understanding in noise for cochlear implant recipients using a spatial noise reduction setting in an off the ear sound processor with directional microphones. Cochlear Implants Int. 2023;24(6):311-324.
* Compared to previous generation Nucleus 7 Sound Processor
^ When the technology becomes available for the Cochlear Nucleus Kanso 3 Nexa Sound Processor, a firmware update to your sound processor will allow you to connect to Bluetooth LE Audio compatible devices.

About MED-EL
We are a Europe-based market leader in implantable hearing solutions, co-founded by
industry pioneers Ingeborg and Erwin Hochmair. It was their ground-breaking research at the Technical University of Vienna that led to the development of the world’s first micro-electronic multi-channel cochlear implant (CI) system, implanted in December 1977, which formed the basis of the modern CIs in use today. Since then, MED-EL have remained innovation leaders, introducing numerous technological breakthroughs throughout our history.
Discover more
Unique Hearing Implants
We understand that choosing a cochlear implant system can be a complicated and emotional decision.
To support you, we have compiled a short, informative 5-minute video. It covers everything you need to know, including who we are, our unique implant, audio processor options, and the dedicated support services we offer. Watch the video now and take the first step towards making an informed decision!

Discover more
Connect With Hearing Implant Users






Hearpeers is a support network for hearing implant users, potential users, parents, and caregivers.
What Can Hearpeers Do for You?
- CONNECT and engage with a supportive community of hearing implant users and receive guidance at every step on your journey to hearing.
- LEARN from those who’ve walked the journey before you. Gain valuable tips, insights, and answers from individuals who share your experiences and can provide relatable guidance.
- HEAR Inspiring stories and immerse yourself in insightful discussions by attending a Hearpeers webinar on a broad range of topics. Visit our Hearpeers events page to register or request a previous webinar recording.
For more information or to be matched with a local Hearpeers mentor, contact Nicola at HearpeersUK@medel.com. Connect with us to enhance your journey to hearing through network support.
Discover more
Complimentary Patient Counselling Service
MED-EL UK & Ireland is excited to announce BeHeard by MED-EL, a free counselling service dedicated to supporting those on their journey to hearing.
The counselling service is designed to provide comprehensive support to adults and families with the help of an accredited therapist. This includes:
the help of an accredited therapist. This includes:
- An initial online assessment to determine individual needs.
- Group or one-to-one counselling sessions.
- Online tools and resources that explore various themes to support their wellbeing.
Discover more
Three Simple Steps Candidate Brochures from MED-EL
Created locally, the packs guide candidates through the decision-making process with 3 steps.
Step 1: Choosing the implant
Step 2: Choosing the audio processor
Step 3: Choosing the connectivity devices
Follow the link below to request a free copy.
Discover more
Connect With Us
Contact our UK and Ireland team:
https://www.medel.com/en-gb/contact-med-el
customerservice@medel.co.uk
0330 123 5601
Ireland queries freephone tel:1800 814131

Oticon Medical www.oticonmedical.com is a global company in implantable hearing solutions, dedicated to bringing the magical world of sound to people at every stage of life. As a member of one of the world’s largest groups of hearing health care companies, we share a close link with Oticon and direct access to the latest advancements in hearing research and technologies. Our competencies span more than a century of innovations in sound processing and decades of pioneering experience in hearing implant technology.
By working collaboratively with patients, physicians and hearing care professionals, we ensure that every solution we create is designed with user needs in mind. We share an unwavering commitment to provide innovative solutions and support that enhance quality of life for people wherever life may take them.
Because we know how much sound matters.

Realising the potential of cell therapy to treat hearing loss
Hearing loss affects >500m people globally, with significant personal and societal impact. Whilst various assistive devices exist, there are currently no treatments that can restore lost hearing.
Rinri Therapeutics is dedicated to developing the world’s first regenerative cell therapies for hearing loss. By replacing the inner ear’s dead or damaged sensory cells that cause sensorineural hearing loss, the company is developing a portfolio of life-changing new approaches. The company is initially focused on neural hearing loss — a critical unmet need with no existing treatment options. By harnessing regenerative cell therapy, Rinri aims to restore auditory nerve function, potentially offering a transformative approach for patients affected by this condition.
Encouraging preclinical data indicate that Rinri’s technology platform has the potential to transform the field. Rincell-1, the company’s first product, is expected to enter clinical trials soon. We’re proud to work closely with patients, their families, and leading doctors to ensure we develop the safest and most effective treatments for severe hearing loss.
As a pioneer in regenerative treatments for sensorineural hearing loss, Rinri is currently the only company specifically addressing neural hearing loss through cell therapy.
For more information:
www.rinri-therapeutics.com
enquiries@rinri-therapeutics.com
+44 [0] 114 222 4330
https://www.linkedin.com/company/rinri-therapeutics